Automotive tools & equipment
HVAC & Industrial supplies
Sales - parts -service
|
|
|
Effects Of Pressure and Temperature on the Boiling Points of Water Last Updated: 11/03/2007 |
|
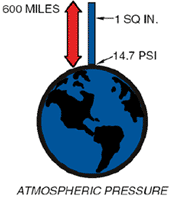
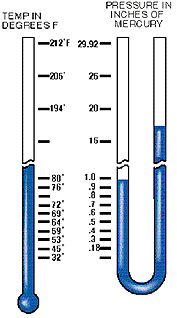
The planet Earth is surrounded by matter in a gaseous state composed of about 78% nitrogen, 31% oxygen and 1% a mix of rare gases. Together they form our atmosphere, which extends approximately 600 miles above the earth and is held to the earth by gravity. Being a gas, the atmosphere has weight, and that weight is measured, as in any fluid whether liquid or gas, in pounds per square inch (psi). If you were to take a square inch column of the air extending six hundred miles above the earth, its weight and pressure exerted on the earth at sea level would be 14.7 lbs. This is called atmospheric pressure . Any pressure above atmospheric pressure is referred to as gauge pressure. Pressures below are referred to as vacuum. This same square inch column of air exerting 14.7 psi can support a one-inch square column of mercury (Hg) 29.92 inches high. This concept can best be understood by comparing it to a teeter-totter. When a one square inch column of Hg 29.92" high is placed on one end of the teeter-totter, and a 14.7 lb weight on the other end, the board will be balanced. Atmospheric pressure decreases at higher elevations. As stated, 600 miles of atmosphere at sea level is equivalent to 14.7 psi and/or 29.92 inch column of mercury (Hg). Going above sea level, to the summit of Mt. Whitney, for example, eliminates some of the 600 miles of atmosphere and, consequently, some of the pressure.
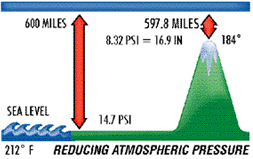 Atmospheric pressure, therefore, governs the boiling point of water. At sea level, where atmospheric pressure is 14.7 psi (29.92" Hg), water boils at 212° F, but on Mt. Whitney, where atmospheric pressure is 8.32 psi (16.9: Hg), water boils at 184° F. The lower the atmospheric pressure is, the lower the boiling point of water. Therefore, if we can significantly reduce the atmospheric pressure inside a sealed refrigerant system, we can vaporize (or boil) moisture at even –90° F. This principle is illustrated in the chart below.
Three ways exist to eliminate moisture from a refrigerant system by the boiling process:
The first two choices are impractical. Thus, a high vacuum pump is an essential aid to every service technician. 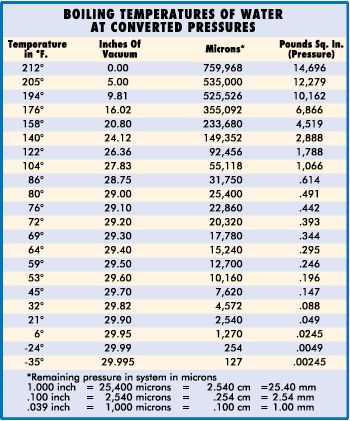 High Vacuum/Deep Vacuum As illustrated above, the purpose of a vacuum pump is to reduce the internal system pressure of a refrigeration/air conditioning system so moisture and other contaminants can be removed. The term "high vacuum" describes a condition where the internal system pressure is extremely low, or close to a perfect vacuum. The higher the vacuum is in a system, the closer the micron reading is to 0 microns. Deep vacuum can be thought of in the same way. The deeper a vacuum is, the closer the micron reading is to 0 microns. "High vacuum" and "deep vacuum" describe the same condition inside a closed system. For refrigeration/air conditioning service applications, high vacuum = good vacuum, or a low micron reading on the system. Selecting High Vacuum Pumps
Chart II shows a vacuum pump capable of eliminating all but one inch of mercury. It is able to remove moisture at an ambient temperature of 80 degrees F or over. While any pump pulling within one inch of atmospheric pressure can eliminate moisture, it must also be capable of holding that vacuum throughout the dehydration process. In addition, it must pull that vacuum on the entire system and not simply at the intake of the pump. Gas Ballast (Vented Exhaust) Pumps The gas ballast or vented exhaust feature on some vacuum pumps permits relatively dry air from the atmosphere to enter the second stage of the pump. This air reduces the compression in the final stage, which helps to prevent moisture from condensing into a liquid and mixing with the vacuum pump oil. Here's a comparison to show how it works: Imagine a damp towel being twisted until water drops out. This can be compared to a high The damp towel comparison also illustrates why this valving feature cannot handle large amounts of moisture. If the towel is almost saturated with water, even the introduction of the completely dry towel will not prevent some of the water from dropping out when the two are compressed. Because of this, some pumps are designed to run with both high internal temperatures (to reduce moisture condensation in the oil) and a gas ballast. Due to the "severe" application of a vacuum pump when used to "boil water," it is necessary to select a quality two-stage model equipped with a gas ballast and high internal running temperature to achieve adequate performance over a long period of time. However, even the best pump must have regular maintenance to perform optimally. Frequent oil changes should be anticipated and considered as the single most important factor in a preventative maintenance program. Remember, even a pump equipped with a gas ballast cannot handle large amounts of moisture without some being condensed into the oil. If allowed to remain inside the pump, this moisture will result in lock ups or loss of efficiency and/or capacity. Normally, oil changes will not be required during a single dehydration job. But it would be well to change oil after each major pump down. This is especially critical when pumping down a system known to be wet or experiencing a compressor burn-out. |
 vacuum pump which is not equipped with a gas ballast. Moisture being pulled from a wet refrigerant system is compressed internally in the vacuum pump and condenses into a liquid. Now imagine the same damp towel entwined with a dry towel and then twisted. It would take a considerable amount of twisting before any water would drop out. Thus the process of the gas ballast arrangement permits the moisture-laden air passing through the pump to mix with relatively dry air to such a degree that compression does not cause condensation.
vacuum pump which is not equipped with a gas ballast. Moisture being pulled from a wet refrigerant system is compressed internally in the vacuum pump and condenses into a liquid. Now imagine the same damp towel entwined with a dry towel and then twisted. It would take a considerable amount of twisting before any water would drop out. Thus the process of the gas ballast arrangement permits the moisture-laden air passing through the pump to mix with relatively dry air to such a degree that compression does not cause condensation.